Hydrogen is a gas with no color, smell, or taste. It is highly flammable and is used as a fuel. Hydrogen is a very important element and it is placed in the first position in the periodic table. We have gathered a complete set of Hydrogen Facts that will help you in learning all about the Hydrogen Element. You are going to learn Hydrogen Element Facts along with its symbol, classification, physical properties, chemical properties, history, discovery, where it is found, industrial production, how to make it at home, usage as fuel, hydrogen bond, its isotopes, and many other interesting and unknown hydrogen facts.
Hydrogen Facts
1. What Is Hydrogen – What Is Hydrogen Gas
- Hydrogen is a chemical element.
- With atomic number 1 and symbol H, it lies on the top of the periodic table.
- Of all the elements of the periodic table, hydrogen is the lightest with a standard atomic weight of only 1.008 u.
- Hydrogen is colorless, tasteless, and odorless at standard temperature and pressure.
- It is non-toxic and non-metal and burns easily.
- Hydrogen is 75% of all the baryonic mass and is the most common element in the Universe.
- Stars are mostly made up of hydrogen.

2. What Is The Symbol For Hydrogen – What Is The Formula Of Hydrogen
- Hydrogen is denoted with the symbol ‘H’.
- Hydrogen gas usually exists as a diatomic molecule and is denoted with the chemical and molecular formula “H₂”.
3. What Does Hydrogen Mean – What Is The Origin Of Hydrogen
- The word hydrogen is derived from two Greek words :
- “hydro” which means water
- “genes” which means the creator
- A French chemist Antoine Lavoisier named it hydrogen because it “creates water” when it is burned.
4. What Does Hydrogen Do – Function Of Hydrogen
- Hydrogen has countless functions. The following are some of the major functions of hydrogen:
-
- Water is made up of Hydrogen and Oxygen, which is vital for the existence of life.
- The biological compounds (proteins, carbohydrates, and lipids) are mostly made up of Hydrogen, Carbon, and Oxygen. These biological compounds are the building blocks of all living organisms.
- Stars use Hydrogen as a raw fuel to produce energy through the process of Nuclear Fusion.
5. Characteristics Of Hydrogen
- Hydrogen is the lightest element with Atomic Number 1, which means it has only one electron and one proton.
- The Atomic Mass of Hydrogen is 1.008.
- It is non-metallic, non-toxic, colorless, odorless, tasteless, and highly flammable gas.
- It is insoluble in water.
- There are 3 isotopes of Hydrogen with atomic masses 1, 2, and 3.
- Hydrogen is the 2nd gas after Helium, the liquefaction of which is extremely difficult.
- Because of having only one electron in the outer orbit, hydrogen shows oxidative (electron-giving) as well as reductive (electron-accepting) properties.
6. History Of Hydrogen – Discovery Of Hydrogen
Who Discovered Hydrogen
- Robert Boyle discovered and separated hydrogen.
- However, it was Henry Cavendish who showed that hydrogen is a separate gas and form water upon burning.
- In the early years of the 15th century, the alchemist Paracelsus observed that when small pieces of iron (iron filings) were added to sulfuric acid, flammable bubbles are formed.
- Later in 1671, Robert Boyle observed the same reaction. He was the first person who separated hydrogen.
- In 1766, Henry Cavendish collected the hydrogen bubbles and revealed that they were different from the other gases. Later he showed that water is formed when this gas burns.
- Antoine Lavoisier then gave it the name Hydro-gen, which means the water-former or water-originator.
When Was Hydrogen Discovered – Where Was Hydrogen Discovered
- Hydrogen was discovered in the year of 1671.
- Robert Boyle was an Irish and grew up in Ireland.
- So hydrogen was probably discovered in Ireland.
How Was Hydrogen Discovered
- Hydrogen was discovered in the metal-acid reaction.
- Robert Boyle did a reaction between dilute acids and iron filings, he found that bubbles were created which were flammable.
- Later, Henry Cavendish collected the gas and showed that water is formed upon the burning of the newly discovered gas.
- The discovery of hydrogen ended the belief in water as a separate element.
7. What Are The Properties Of Hydrogen – Hydrogen Properties – Properties Of Hydrogen Gas
- The properties of hydrogen fall into two categories:
- Physical properties
- Chemical properties
Physical Properties Of Hydrogen Gas
- The physical properties of an element mean its characteristics that differentiate it from other elements, and which can be measured without any change in the chemical nature of that element.
- The following are the physical properties of hydrogen:
| Color | Colorless |
| Test | No test |
| Smell | No smell |
| State of matter | Gas |
| Density | 0.00523 Ib/ft³ (0.08378 kg/m³) |
| The temperature at which it changes to liquid | -252.9℃ |
| The temperature at which liquid Hydrogen changes to solid (freezing point) | -259.2℃ |
| The heat of evaporation (H₂) | 0.904 KJ/mol |
| Solubility | Slightly soluble in water, alcohol, and some other liquids |
| Molar heat capacity (H₂) | 28.836 J/(molᐧK) |
Hydrogen Chemical Properties – What Does Hydrogen React With
- The following are the major chemical properties of Hydrogen:
- Hydrogen exists as a diatomic molecule (H₂). The bond dissociation energy for the Hydrogen molecule (the energy required to break the bond between the two atoms of hydrogen OR the measure of the strength of the chemical bond between two hydrogen atoms) is 436 KJ/mol, which is extremely high.
H₂ + 436 KJ/mol (energy required) → H + H
- Hydrogen is highly flammable and burns in the air. The burning reaction is known as combustion and the heat released is known as the heat of combustion. The heat of combustion for one mole of H₂ is 286 KJ.
2H₂(g) + O₂(g) → 2H₂O(l) +572 KJ (286 KJ/mol)
- Despite its stability, molecular Hydrogen (H₂) reacts with many elements and molecules. It reacts with halogens (Florine, Chlorine, Bromine, ….) in the dark and in the presence of light, sunlight, Ultraviolet light, or temperature and forms Hydrogen Halides.
H₂(g) + F₂(g) → 2HF
H₂(g) + Cl₂(g) → 2HCl
H₂(g) + Br₂(l) → 2HBr
H₂(g) + I₂(s) → 2HI
- Molecular Hydrogen (H₂) reacts with nitrogen and form ammonia (NH₃) in the presence of iron or nickel catalyst and at a temperature of 410℃. The reaction is exothermic and releases 92 KJ/mol energy. This reaction is known as the Haber Process on the industrial level and is the major process for the production of ammonia.
N₂(g) + 3H₂(g) → 2NH₃ (g)
- Hydrogen reacts with metal ions (such as Pd²+, Fe³+) and metal oxides as a reducing agent.
Fe₂O₃ +3H₂(g) → Fe + 3H₂O
PdCl₂ + H₂(g) → Pd + 2HCl(g)
- Hydrogen reacts with Alkaline metals (Lithium, Sodium, Potassium, ….) and Alkaline-Earth metals (Magnesium, Calcium, …) to form metal-hydrides.
2M + H₂ → 2MH
2Na + H₂(g) → 2NaH
M + H₂ → MH₂
Ca + H₂(g) → CaH₂
- The reaction of Hydrogen with organic compounds is used to produce a large variety of other compounds. The most common examples are the conversion of; vegetable oil into ghee, alkene, and carbon monoxide into aldehydes and aldehydes into alcohols.
vegetable oil +H₂ + (Ni catalyst) → Ghee
H₂ + CO + RCH=CH₂ → RCH₂CH₂ CHO (aldehyde)
RCH₂CH₂ CHO +H₂ → RCH₂CH₂CH₂OH (alcohol)
8. Ionization Energy Of Hydrogen Atom – What Is The Ionization Energy Of Hydrogen
- The ionization energy means the lowest amount of energy required to remove an electron from a gaseous atom, ion, or molecule. It is denoted with the symbol Ei.
- For hydrogen, it is the energy required to remove the only electron orbiting the nucleus.
- The ionization energy of hydrogen is 2.18 × 10−18 joules.
9. What Is The Melting Point Of Hydrogen – Hydrogen Melting Point
- The melting point of hydrogen is -259.16℃ (-434.49℉).
- In Kelvin, it is 13.99 K.
10. What Is The Boiling Point Of Hydrogen – Hydrogen Boiling Point
- The boiling point of hydrogen is -252.879℃ (-423.182℉).
- In Kelvin, it is 20.271 K.
11. What Is The Density Of Hydrogen – Hydrogen Density
- At standard temperature and pressure, the density of gaseous hydrogen is 0.08988 g/L.
- The density of solid Hydrogen at the melting point is 0.0763 g/cm³.
- When liquid, the density of hydrogen at the boiling point is 0.07099 g/cm³.
12. Hydrogen State Of Matter
- Hydrogen exists in the gaseous state of matter at standard temperature and pressure.
13. Heat Capacity Of Hydrogen
- Heat capacity means the amount of heat required to produce one unit change in the temperature of a given mass of material.
- The heat capacity of one mole of hydrogen (H₂) is 28.836 J/(mol.K).
14. Specific Heat Capacity Of Hydrogen
- Specific heat capacity of material means, the heat capacity of a sample of the material divided by the mass of the sample.
- In informal words, it is the amount of heat given to one unit mass of a material to cause one unit increase in its temperature.
- The SI unit of specific heat capacity is Joule/Kelvin and Kilogram.
- The specific heat capacity of Hydrogen is 14300 J/(K.kg).
15. Critical Temperature Of Hydrogen
- In thermodynamics, the critical temperature is the temperature above which the liquid phase of a substance can not exist, no matter how much pressure is applied.
- On the phase diagram, the critical point is a point at which the gas and liquid phases of a substance have similar densities and they can not be distinguishable from one another.
- The critical temperature of Hydrogen is -239.95 ℃ (33.20 K).
16. Compressibility Factor Of Hydrogen
- A correction factor that measures the deviation of a real gas from ideal gas behavior is known as the compressibility factor, the compression factor, or the gas deviation factor.
- It is denoted with the symbol “Z”.
- In statistical mechanics, it is described as:
Z= PV/ nRT
- While ‘P’ is pressure, ‘V’ is volume, ‘n’ is the number of moles of gas, ‘R’ is the gas constant, and ‘T’ is the absolute temperature.
- For an ideal gas, the value of Z is equal to 1.
- For Hydrogen, the compressibility factor at 25℃ (298.15 K) temperature and different pressures are shown in the following table:
| Pressure (atm) | Compressibility factor (Z) |
| 3.5129 | 1.0021 |
| 4.6129 | 1.0027 |
| 5.4954 | 1.0032 |
| 7.2187 | 10042 |
| 8.6021 | 1.0050 |
| 11.306 | 1.0066 |
| 17.732 | 1.0103 |
| 27.872 | 1.0163 |
| 52.613 | 1.0312 |
- As the pressure increases, the value of Z increases.
17. What Does Hydrogen Look Like – Hydrogen Appearance
- Hydrogen gas has no color, no smell, and no test at standard temperature and pressure.
- When burns in the air, its flames are invisible.
18. What Does A Hydrogen Atom Look Like
- A hydrogen atom is the simplest of all the atoms of other elements.
- There is only one electron in the hydrogen atom, which is orbiting a single proton in the nucleus.
19. What Type Of Element Is Hydrogen
- Hydrogen is a “Non-metal” type of element.
- It is also included in the reactive nonmetals along with Carbon, Nitrogen, Phosphorous, Oxygen, Sulphur, Selenium, Florine, Chlorine, Bromine, and Iodine.
20. Where Is Hydrogen Found – Occurrence Of Hydrogen
- In all the Universe, Hydrogen is the most abundant element.
- Sun, planet Jupiter, and most of the stars are mostly composed of Hydrogen.
- All the baryonic matter (about all types of matter we encounter or experience in our daily lives) is 75% made up of Hydrogen.
- On the Earth’s surface, it is found in huge quantities in the form of water.
- Hydrogen is one of the basic units of the biological compounds; the building blocks of all living organisms.
Where Is Hydrogen Found On Earth
- Hydrogen is one of the building blocks of about any type of material found on the Earth, such as hydrocarbons, water, all the living organisms, and Earth’s crust.
21. What Is Hydrogen Made Of – Hydrogen Composition
- The composition of a hydrogen atom is one electron and one proton.
22. How Is Hydrogen Made – How To Produce Hydrogen
- There are several ways of hydrogen production. The following are the major methods of hydrogen production:
Steam Reforming or Steam Methane Reforming Method
- In this method, methane gas is heated between 700-1100 ℃ in the presence of steam and nickel catalyst.
- The molecules of methane gas split and form carbon monoxide (CO) and hydrogen (H₂).
- Along with steam, the carbon monoxide gas is then passed over oxides (usually iron oxide) that undergo the water gas shift reaction and more amount of hydrogen gas is obtained.
CH₄ +H₂O → CO + 3H₂
CO + H₂O → CO₂ + H₂ (water gas shift reaction)
Partial Oxidation of Fossil Fuels
- In this method, natural gas or other hydrocarbons are partially oxidized to produce hydrogen.
- A mixture of fuel-oxygen (fuel-air) is partially combusted, which leads to the formation of Syngas (a mixture of H₂, CO, and some CO₂).
- Syngas has a rich amount of hydrogen, which is obtained along with carbon monoxide through the water gas shift reaction.
CxHy + x/₂ O₂ → xCO + y/₂ H₂
C₁₂H₂₄ + 6O₂ → 12CO + 12H₂
C₂₄H₁₂ + 12O₂ → 24CO + 6H₂
Plasma Reforming
- This process is also known as Kvaerner Process or Kvaerner carbon black and hydrogen Process.
- In this method, liquid hydrocarbons are heated at around 1600℃ in a plasma burner, which decomposes into carbon and hydrogen.
CxHy → xC + y/₂ H₂
- In this method, a complete and efficient transformation of hydrocarbons into pure carbon and hydrogen occurs.
Coal Gasification
- In this method, coal is heated along with air and steam to produce syngas.
3C (coal) +H₂O +O₂ → H₂ +3CO
- Hydrogen is then obtained through the water gas shift reaction.
CO +H₂O → CO₂ +H₂
Electrolysis of Water
- In this method, an electric current is used to split water into oxygen and hydrogen.
- If the electricity used in this process is obtained through renewable sources (such as wind or solar), the hydrogen produced would be also considered renewable.
Renewable Liquid Reforming
- In this method, renewable liquid fuels like ethanol are reacted with steam at high temperatures to produce hydrogen.
Fermentation
- In this method, biomass is converted into sugar or glucose-rich matter.
- This matter can be then fermented to produce hydrogen.
- Two types of fermentation methods are used, that is;
- Dark fermentation (do not require light energy)
- Photo-fermentation (require light energy and can only proceed in light)
23. How To Make Hydrogen Gas At Home
- The following is the easiest method of making hydrogen at home:
- Take two paper clips and unbend them.
- Connect the unbent paper clips to the terminals of the battery.
- Place the free ends of wires (unbent paper clips) in a water container.
- Bubbles would be produced near both the wires.
- There would be more bubbles near one wire, which is hydrogen gas.
- If you want to check, then light a match over the container and you will see that hydrogen bubbles burn.
- The bubbles at the other wire are impure oxygen.
- If you want to collect hydrogen gas, then take a water-filled tube or jar and invert over the hydrogen-producing wire.
- Water should be taken in the tube or jar to prevent air from accumulating in it and to take pure hydrogen.
24. What Is Hydrogen Used For – Uses Of Hydrogen
Common Uses Of Hydrogen
- Some of the most common uses of hydrogen are:
- In the hydrogenation of vegetable oils into ghee
- Welding
- As a coolant in electrical generators at power stations
- Extraction of metal from ores
- In the production of HCl
Industrial Uses Of Hydrogen
- The following are some of the major uses of hydrogen on the industrial level:
Ammonia production
- Hydrogen is used for the production of ammonia through the Haber process.
- In this process, atmospheric nitrogen is fixed, to which hydrogen reacts and forms ammonia.
- Ammonia is used as a nitrogen fertilizer, either in pure form or as urea or ammonium nitrate.
- Most of the ammonia is produced through the Haber process all over the world.
Hydrocracking and refining of petroleum
- Hydrogen is used to convert fractions of heavy petroleum into lighter ones through a process known as hydrocracking.
- In this process, C-C bonds are broken in the presence of hydrogen.
- In this process, the major functions of Hydrogen are:
-
- To prevent the formation of polycyclic aromatic compounds
- To reduce impurities
- To reduce the formation of tar
- To prevent the gradual accumulation of coke on the catalyst
- To convert the nitrogen and sulfur compounds (present in the feedstock) to ammonia and hydrogen sulfide
- To achieve fuel with high cetane number (a performance or quality measurement of diesel fuel)
- Through hydrocracking, up to 265×10⁶ tons of petroleum were processed in the year 2010.
Hydrogenation of vegetable oil
- Hydrogen is used on the industrial level to convert vegetable oil into ghee.
- In this process, hydrogen is added to the carbon-carbon double bond (C=C) found in the fatty acids of vegetable oils.
- Hydrogen is used as a tracer gas (in pure form or mixed with nitrogen) to detect minute leaks in chemical, automotive, power generation, and telecommunication industries.
Other industrial uses of hydrogen are:
- Metal alloying and other metalworking
- As a protective or inerting gas in the production of flat glass
- As a carrier and inerting gas in the electronics industry, usually for the processes of deposition, etching, cleaning, reduction, etc.
- For the direct reduction of iron ore (removing oxygen from the iron ore)
Hydrogen Uses In Everyday Life
- The use of water is the major use of hydrogen in everyday life.
- Hydrogen is used in fuel cells to generate power.
- Hydrogen gas is used as a packaging gas and is an authorized food additive (E949) because of its anti-oxidizing properties.
- The International Temperature Scale of 1990 (ITS-90) uses the triple point of hydrogen as a defining fixed point at 13.8033 Kelvin.
25. Where Is Hydrogen On The Periodic Table – Hydrogen Periodic Table
- On the periodic table, hydrogen lies at the top of the Group 1 elements.
- However, it is not included in the “alkali metals”, a term for the members of Group 1 elements.
26. What Group Is Hydrogen In – Hydrogen Group
- Hydrogen is placed in Group-Ⅰ of the periodic table ‘Hydrogen and Alkali Metals’.
27. What Family Is Hydrogen In – Hydrogen Element Family – What Family Does Hydrogen Belong To
- Of all the elements of the periodic table, hydrogen is a special element.
- It is placed on the top and does not belong to any family.
28. What Is Hydrogen Classified As – Hydrogen Classification
- Based on physical properties, hydrogen is classified as a ‘non-metal’ element.
- Hydrogen is placed in Group-Ⅰ (the alkali metals), however, it is not an alkali metal.
- It is placed in this group because there is only one electron in the hydrogen atom while alkali metals also have only one electron in their outermost orbits.
29. What Is The Atomic Mass Of Hydrogen – What Is The Mass Of A Hydrogen Atom
- The atomic mass (or atomic weight) of hydrogen is 1.00784 u.
30. What Is The Molar Mass Of Hydrogen – Hydrogen Molar Mass
- The molar mass of hydrogen is 1.00784 u.
31. What Is The Molecular Mass Of Hydrogen – Hydrogen Molecular Mass
- The molecular mass of hydrogen is 2.016 g/mol.
32. What Is The Atomic Number Of Hydrogen – Hydrogen Atomic Number
- The atomic number of an element means, the number of protons in the nucleus or the number of electrons in the individual atoms of that element.
- As there is only one proton and one electron in the hydrogen atom, the atomic number of hydrogen is 1.
33. How Many Electrons Does Hydrogen Have
- Hydrogen atoms have only one electron.
How Many Valence Electrons Does Hydrogen Have
- There is only one electron in the hydrogen atom orbiting the nucleus.
- So this only electron is the valence or outermost.
34. How Many Hydrogen Atoms Are In A Molecule Of Water
- The formula of water is H₂O, which means there are two atoms of hydrogen and one atom of oxygen in a water molecule.
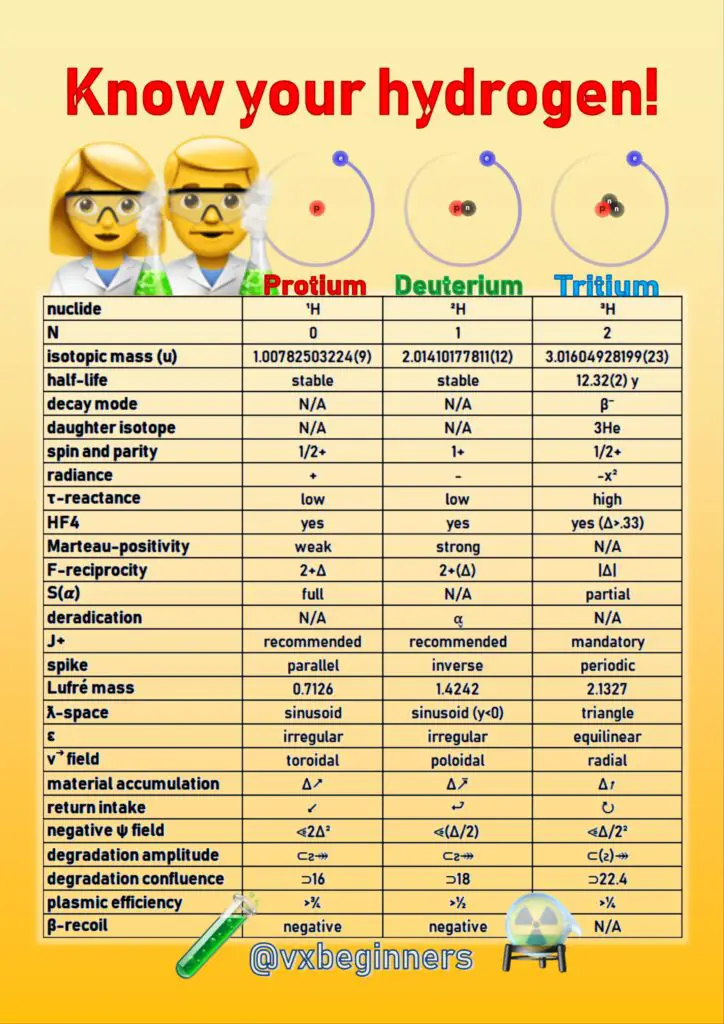
35. What Are Hydrogen Isotopes
- The isotope of an element means its various forms which have the same atomic number (same number of electrons and protons) but a different number of neutrons, and consequently a different atomic mass.
- There are three isotopes of hydrogen:
- Protium (¹H)
- Deuterium (²H)
- Tritium (³H)
What Is Protium
- Protium (¹H) isotope is the most common form of hydrogen.
- It takes about 99.98% of the entire hydrogen in the universe.
- The name protium is given to it because there is only one proton in its nucleus.
- The atomic mass of protium is 1.00782504 u.
What Is Protium Used For
- Protium is the common form of hydrogen and has a wide range of uses, which are described above.
What Is Deuterium
- Deuterium (²H) is the isotope of hydrogen usually denoted with the symbol D.
- There is one proton and one neutron in the nucleus of deuterium.
- Deuterium makes approximately 0.0026 to 0.0184% of all the hydrogen atoms found on the Earth.
- It is very little in the Earth’s gaseous hydrogen and is naturally abundant in seawater, about one atom in 6420 of hydrogens.
- Deuterium is not harmful to living things, as it is not radioactive.
- It can also form water molecules, which is known as heavy water (D₂O).
What Is Deuterium Used For
- The following are some of the uses of deuterium:
- Deuterium is used in nuclear reactors as heavy water (D₂O) to moderate neutrons.
- Liquid D₂ is also used in cold sources in research reactors, where it functions to moderate the energies and wavelengths of neutrons to a very lower level suitable for scattering experiments.
- In nuclear fusion reactor designs, deuterium is the most commonly used nuclide in combination with tritium.
- In the Nuclear Magnetic Resonance Spectroscopy of hydrogen (proton NMR), deuterium is most commonly used because of its spin properties, which are different from the common light hydrogen present in organic molecules.
- It is used as a non-radioactive and stable isotopic tracer in environmental science, chemistry, and biochemistry. Such as, in the doubly labeled water test.
- Currently, it is also used in drugs and reinforced essential nutrients.
What Is Tritium
- Tritium (³H) is another isotope of hydrogen usually denoted with the symbol T.
- There are one proton and two neutrons in the nucleus of the tritium.
- It is radioactive and has 12.32 years of half-life.
- Tritium is very rare on Earth.
- It is found only in trace amounts in the atmosphere where it is formed when the cosmic rays interact with atmospheric gases.
- It can be produced in nuclear reactors when lithium metal or lithium-bearing ceramic pebbles are exposed to radiation.
What Is Tritium Used For
- Tritium is used as a radiotracer (or radioactive tracer).
- In watches and other instruments, it is used in radioluminescent light sources.
- In nuclear reactors, it is used as a fuel along with deuterium for nuclear fusion reactions.
36. What Is The State Of Hydrogen – Hydrogen State
- At standard temperature and pressure, hydrogen exists in a molecular gaseous state (H₂) on the Earth.
- While it is found in the atomic (H) and plasma states (the electron and proton of hydrogen are not bound together) throughout the universe.
37. What Is Hydrogen Energy – What Is Hydrogen Fuel – Hydrogen Energy Facts
- Hydrogen fuel is a zero-emission (emits no waste product that pollutes the climate) alternative source of energy.
- It is the cleanest fuel and produces only water when burned with oxygen.
2H₂(g) + O₂(g) → 2H₂O(l) + Energy
- The reaction is exothermic and the energy released enables hydrogen to act as a fuel.
- Like electricity, hydrogen is considered as an energy carrier, because it must be created from a primary energy source, such as fossil fuels, solar energy, biomass, etc.
- In 2018, about 95% of the hydrogen was produced from fossil fuels through the methods of steam reforming or partial oxidation of methane and coal gasification. While only a small quantity (about 5%) was produced through other methods, such as water electrolysis and biomass gasification.
38. Application Of Hydrogen Fuel
- Hydrogen fuel is commercially used in fuel cell vehicles, such as fuel cell buses and passenger cars. Currently, there are more than 30 commercial hydrogen stations in California state, USA.
- Hydrogen is also used as rocket fuel for the propulsion of spacecraft.
39. What Type Of Energy Is Released By Hydrogen Fuel Cells
- Electrical energy is released by hydrogen fuel cells.
What Is Hydrogen Energy Used For – Uses Of Hydrogen Energy
- Hydrogen energy is used as:
- An attractive fuel (with zero-emission) option for transportation.
- To generate power, electricity, or heat to provide power in buildings and other places that are not connected to electric power stations.
- To power the spacecraft’s electric system
40. What Is A Hydrogen Bond – Hydrogen Bond Definition
- A hydrogen bond is an electromagnetic attractive interaction between the atoms of two polar molecules; one is an electron-rich electronegative atom with a partial negative charge and a lone pair of electrons (such as O, N, F), and the other electron-deficient hydrogen atom with a partial positive charge.
What Makes A Hydrogen Bond
- Unequal charge distribution in a molecule leads to the formation of a hydrogen bond.
- The molecules are said to be polar if one atom in it has a partial negative charge and the others have a partial positive charge.
- For example, if we look at the water molecule where two hydrogen atoms share the electrons of an oxygen atom. So the total number of protons in this molecule is 10 (8 from oxygen and two from both hydrogens) and the number of total electrons is 10 in which four (two from oxygen and two from both hydrogen) are shared between oxygen and hydrogens. While 6 electrons in the oxygen are unshared. So this molecule is electrically neutral. However, the electrons are not equally shared in the molecule and so the molecule has a partial negative side with a high electron density (oxygen) and a partial positive side with a lower electron density (hydrogens).
- When coming together, the positively charged end of one water molecule weakly attaches to the negative end of the adjacent water molecule. That is, the partially positive hydrogen of one molecule makes a weak interaction with the partially negative oxygen of the other molecule.
What Type Of Bond Is A Hydrogen Bond
- A hydrogen bond is an intermolecular electromagnetic attraction.
- It is a special type of “dipole-dipole interaction.
What Elements Can Hydrogen Bond
- Many elements can make hydrogen bonds.
- The second-period elements, nitrogen (N), oxygen (O), and fluorine (F) are particularly known for making hydrogen bonds.
Where Are Hydrogen Bonds Found
- Hydrogen bonds are found in many compounds.
- The most common example of a hydrogen bond is found in water.
- Other examples of small molecules that have hydrogen bonding are ammonia and hydrogen fluoride.
- The most common polymers that have hydrogen bonding are DNA, proteins, and cellulose. The characteristic three-dimensional structure (secondary structure) of proteins and the folding of DNA into specific shapes is due to hydrogen bonding. The specific structure and appearance of cellulose polymers in cotton and flax are also due to hydrogen bonding.
- The well-known examples of synthetic polymers having hydrogen bonds are nylon and aramid fiber.
When Does Hydrogen Bonding Occur
- Hydrogen bonding occurs when a hydrogen atom bonded with a strongly electronegative atom exists in the proximity of another strong electromagnetic atom having a lone pair of electrons.
Hydrogen Bond Structure – What Does A Hydrogen Bond Look Like
Facts About Hydrogen Bonds
- Hydrogen bonds occur when a hydrogen atom attached with a strongly electronegative atom in a molecule comes near the strong electronegative atom of another molecule. The electronegative atoms are usually nitrogen, oxygen, and fluorine.
- The strength of hydrogen bonding varies from weak (1 to 2 KJ/mol) to strong (161.5 KJ/mol).
- The compounds of N, O, and F have many anomalous chemical and physical properties, most of which are due to hydrogen bonding.
- The high boiling point of water (100℃) is due to intermolecular hydrogen bonding.
- Hydrogen bonding helps to create an open hexagonal lattice when the water freezes and has a strong influence on the crystal structure of ice. The orientation of hydrogen bonds pushes apart the water molecules upon freezing. That is why ice has a lower density than liquid water and floats on the surface of liquid water.
- The secondary structure of proteins (ɑ-helix and ?-pleated sheets) is due to hydrogen bonding.
- Hydrogen bonding is also responsible for the special bent and folded structure of the DNA polymer.
41. What Does Hydrogen Do For The Body
- Hydrogen performs a lot of functions in the human body. The following are some of the functions hydrogens do for the body:
- Hydrogen is one of the constituent elements of proteins, carbohydrates, and lipids. These compounds are the primary building blocks of the cells of all living organisms, and so of the human body. These compounds are also an important part of the human diet.
- Water is a vital resource for the survival of humans, which is made up of two hydrogens and one oxygen. All the organs, tissues, and cells in the human body use water to maintain its metabolic functions, regulate temperature, and remove toxins and wastes. Water also keeps the immune system of the human body alert and active.
- Hydrogen is a reducing agent and acts as an antioxidant in the cells. It selectively reduces free radicals. In such a way, it protects the cells from damage and so the human body from aging.
42. What Is Alpha Hydrogen – What Is Alpha Hydrogen Atom
- Inorganic molecules, alpha hydrogen is the hydrogen atom that is attached to the alpha carbon.
- While alpha carbon is the carbon that is directly attached to the functional group.
- For example:
43. What Is Antihydrogen
- Antihydrogen is the antimatter equivalent or counterpart of hydrogen.
- It is denoted with a symbol (⁻H).
- The atom of antihydrogen is made up of an antiproton and antielectron (positron).
44. What Is Heavy Hydrogen
- A deuterium isotope of hydrogen is also known as heavy hydrogen.
45. What Is Liquid Hydrogen
- The liquid form of elemental hydrogen is known as liquid hydrogen.
- It is denoted with the symbol LH2 or LH₂.
- Hydrogen requires to be cooled below its critical point of 33K (-240.15 ℃) to convert into liquid form.
- To obtain fully liquid hydrogen without boiling at atmospheric pressure, H₂ must be cooled to 20.28K (252.87 ℃).
- Liquid hydrogen is usually used to store hydrogen in concentrated form for subsequent use.
- Thermally insulated and pressurized containers are used to maintain it in the liquid state.
What Is Liquid Hydrogen Used For
- The most common use of liquid hydrogen is as a fuel for rockets.
- It is also used as fuel for fuel cells or internal combustion engines. Various types of submarines (Type 212 and Type 214) and hydrogen vehicles are built using liquid hydrogen as fuel.
- Liquid hydrogen is also used for cooling neutrons that are used in neutron scattering.
- Liquid deuterium was used for nuclear fusion in Ivy Mike, the first thermonuclear bomb.
46. What Is Hydrogen-Rich Water – What Is Hydrogen Water
- Hydrogen water or hydrogen-rich water is water that has dissolved hydrogen gas (just like carbonated water which has dissolved CO₂).
- As hydrogen is a tasteless and odorless gas, hydrogen water also has no taste or smell.
- It is obtained by dissolving gaseous hydrogen (H₂) in water under pressure.
- Usually, 7.0 mg of H₂ is dissolved in one liter of water.
What Are The Benefits Of Hydrogen Water
- The proponents of hydrogen water claim that it has many health benefits, such as working as an antioxidant, reducing inflammation, providing neuroprotection in various diseases, minimizing the risk of metabolic syndrome, and lowering the side effects associated with the radiation treatment of cancer.
- However, there is very limited scientific evidence about the health benefits of hydrogen-rich water in humans.
47. What Is Molecular Hydrogen
- Molecular hydrogen (H₂) is a molecule of two hydrogen atoms.
- Usually, hydrogen gas exists in the form of molecular hydrogen.
48. What Is Power Of Hydrogen
- In chemistry, the power of hydrogen is a scale used to measure the acidity or alkalinity (basicity) of an acid-base solution.
- It is denoted with the symbol pH.
- At room temperature, pure water is neutral (neither basic nor acidic) with a pH value of 7.
- While acidic solutions have a lower pH (below 7) and basic solutions have higher pH (above 7).
- The pH scale is a logarithm and inversely shows hydrogen ions concentration in a solution.
pH = -log₁₀ [H+]
- A lower pH value means, a high concentration of hydrogen ions.
- A higher pH means, a low concentration of hydrogen ions.
49. What Is Nascent Hydrogen – Nascent Hydrogen Meaning
- In chemistry, a nascent state is an absolute theory that refers to an element in an instance of its formation or liberation.
- Nascent hydrogen is a concept that was once cited to explain the reactions of dissolving metals.
- This special state of hydrogen was postulated because H₂ does not react with organic compounds.
- Now, it is known that dissolving-metal reactions occur at the surface of the metal and so the concept of nascent hydrogen is discounted.
50. Cool Facts About Hydrogen – Fun Facts About Hydrogen
- Sun and other stars are mainly composed of hydrogen.
- Hydrogen is the only existing element without neutrons.
- Hydrogen makes up about 10% of the total body weight of humans and other organisms, mainly in water, proteins, carbohydrates, and lipids.
- The density of liquid hydrogen is the lowest of all the liquids.
- NASA uses liquid hydrogen as a fuel for spacecraft propulsion.
51. Interesting Facts About Hydrogen – Unknown Facts About Hydrogen
- Before the discovery of Hydrogen, water was believed to be a separate element.
- Hydrogen is an element that is essential for all kinds of life. In the form of water, it is present in almost all molecules in living organisms.
- Hydrogen makes up around 75% of all the atoms in the universe.
- It is believed that hydrogen was one of the three elements produced during the Big Bang; the other two were lithium and helium.
- According to the principles of chemistry, hydrogen is 14 times lighter than air.
- The density of the solid, crystalline hydrogen is the lowest of all the crystalline solids.
- Most of the energy our planet earth receives originates from hydrogen; nuclear fires in the sun convert hydrogen into helium.
- As a byproduct of chemical reactions, hydrogen had been produced long before it was discovered as an element.
- Hydrogen is the main constituent of the gas giants (such as Jupiter).
- Liquid hydrogen is extremely cold and its contact with the skin can cause severe frostbite.
52. Is Hydrogen Harmful To Humans
- Hydrogen is generally not harmful to humans.
- However, it is highly flammable and burns with an invisible flame, and can cause accidental burns.
- Liquid hydrogen is cold to such an extreme that its slight contact with the skin can cause severe frostbite.
- Hydrogen has no smell or test, and breathing it in its pure form can cause asphyxiation (suffocation).